What is the EDTA charge
EDTA is a polydentate ligand with multiple functional groups, which affects the charge characteristics of the whole molecule. The following section explains in detail the charge of EDTA and the mechanism of its formation.
Content Tables
1. Structure of EDTA.
EDTA has the molecular formula C10H14N2O8 and contains in its structure:
- Two amino groups (-NH2): these can be protonated under certain conditions with a positive charge.
- Four Carboxylic Acid Groups (-COOH): these carboxylic acid groups can lose protons (H⁺) in solution and transform into carboxylic acid anions (-COO-) with a negative charge.
2. Electric charge state.
At physiological pH (about 7.4), most of the carboxylic acid groups of EDTA lose their protons, while the amino groups maintain their protonated state. Below are the charge states of EDTA at different pH conditions:
- Acidic conditions (low pH):
- Most of the carboxylic acid groups (-COOH) are in the unprotonated state and EDTA may remain relatively neutral or weakly positively charged.
- Neutral to alkaline conditions (pH 7 and above):
- Three of the four carboxylic acid groups usually lose their protons to form three negatively charged carboxylic acid anions (-COO-).
- The two amino groups (-NH2) remain protonated to form the positively charged amino group (-NH3+).
In this state, EDTA will have an overall charge of -3. This is because:
- The negative charge comes from the deprotonation of the three carboxylic acid groups.
- The positive charge comes from the protonation of the two amino groups.
3. Ionic form of EDTA.
EDTA can exist in different ionic forms, the most common and stable being its disodium salt form (Na2EDTA), in which EDTA is i.e. saturated and electrically neutral, and the disodium ion neutralizes the negative charge generated by EDTA. The overall structural formula is:
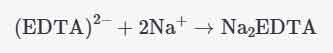
In this form, EDTA remains strong as a ligand and can form stable complexes with a wide range of metal ions.
4. Importance in applications.
The charge characteristics of EDTA are critical for its use as a drug, anticoagulant, and chemical analytical reagent. This charge state affects its ability to bind metal ions, allowing it to exhibit good stability and chelating properties in aqueous solutions.
Summary.
The charge properties of ethylenediaminetetraacetic acid (EDTA) arise mainly from the protonation and deprotonation reactions of its amino and carboxylic acid groups. Under physiological conditions, EDTA is usually negatively charged (overall charge of -3), while binding to sodium ions to form neutral salts, enhancing its stability and biocompatibility in aqueous solutions. Through this charge mechanism, EDTA is able to act as an effective ligand to form complexes with metal ions, which are widely used in industry, agriculture and medicine.
