EDTA-CuNa2, EDTA Copper Salts
CAS No.: 14025-15-1
Molecular Formula: C10H12N2O8CuNa2
Molecular Weight: 397.7
Structural Formula:
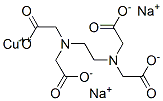
Properties:
EDTA-CuNa2 is a kind of stable water-soluble metal chelate; copper exists in a chelated state. EDTA-Cu can chelate multivalent ferric ions.
Specifications:
| Item | Index |
|---|---|
| Appearance | Blue powder |
| Copper content | 15.0±0.5% |
| pH value (1% aqueous solution) | 6.5±0.5 |
| Water-solubility | About 1200 g/L (20℃), about 1700 g/L (80℃) |
| Bulk density | About 625kg/m3 |
Preparation method:
The preparation of EDTA-Cu is generally achieved by reacting ethylenediaminetetraacetic acid (EDTA) with copper salt and sodium salt under appropriate conditions. The reaction process requires a high reaction temperature and neutral or weakly alkaline reaction conditions.
Usage:
EDTA-CuNa2 is used as a trace element in agriculture. This EDTA copper salt is hard to be biodegraded. The COD (Chemical Oxygen Demand) value is about 560mg/g.
Used as a separating agent, EDTA-CuNa2 also can be used for foliar fertilizers.
Packing and Storage:
25kg woven bag. Stored in a cool dry place, it must be tightened after being opened.
Safety and hazards:
When using EDTA copper salt, it is important to pay attention to coordinating the experimental conditions and controlling the temperature to avoid the generation of toxic gases. It is necessary to wear appropriate personal protective equipment, such as chemical-resistant gloves, goggles, and a respirator.
Keywords:
EDTA-CuNa2, EDTA-Cu, EDTA cupric salt.
-
EDTA Series
- EDTA Acid
- EDTA-Na2
- EDTA-Na4
- EDTA-CaNa2
- READ MORE...
-
Organophosphoric
- ATMP
- BHMTPMPA
- DTPMPA
- EDTMPA
- HEDP
- READ MORE...
-
Other Chelating Agent
- DTPA
- NTA.Na3
- DTPA.Na5
- READ MORE...


