Ethylene Diamine Tetraacetic Acid Zinc Disodium (EDTA-ZnNa2)
CAS No.: 14025-21-9
Molecular Formula: C10H12N2Na2O8Zn
Molecular Weight: 399.59
Structural Formula:
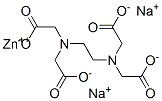
Properties:
EDTA-ZnNa2 is used as a trace element in agriculture.
Environmental Aspects:
Biodegradability: Difficult.
COD (Chemical Oxygen Demand) Value: 555 mg/g.
Specifications:
| Item | Index |
| Appearance | White Powder |
| Zinc Content | 15.0±0.5% |
| pH Value (1% aqueous solution) | 6.0±7.0 |
| Dissolubility | About 1000 g/L (20 ℃), about 1200 g/L (80 ℃) |
| Bulk Density | About 625 kg/m3 |
Usage:
EDTA-ZnNa2 is a stable water-soluble metal chelate, and zinc exists in a chelated state. EDTA-ZnNa2is used as a separating agent.. EDTA-ZnNa2 is a kind of stable water-soluble metal chelate. EDTA-ZnNa2 can chelate multivalent ferric ions. EDTA-ZnNa2can be used for foliar fertilizers.
Packing and storage:
25kg woven bag. Stored in a cool, dry place, it must be packaged after opening.
Keywords:
EDTA-ZnNa2; EDTA-Zn; EDTA Zinc Salt; EDTA
EDTA and Zn2+
1. What is the coordination number of Zn2+ when EDTA forms a complex with
Zn2+?
EDTA is an amino carboxylic chelating agent with six coordinating atoms (two amine
nitrogen and four carboxyl oxygen donating lone pair electrons). It can form 5-atom ring
stable 1:1 chelates with metal ions. The coordination
number of the central ion (Zn2+) is 6. Regular octahedral structure.
2. When EDTA titrates Zn2+, why should NH3-NH4Cl
be added?
NH3-NH4Cl is added as a buffer to stabilize the pH of the
solution.
Zn2+ was titrated with EDTA. The metal indicator is first added to the
zinc solution. The metal indicator is first added to the zinc solution. Then, the zinc
reacts with the indicator to form a complex, and the
color changes. Metal indicators are generally weakly acidic, with high sensitivity and
obvious color reaction. But at the same time, it also has a certain pH range. Beyond
this range, the color change will not be obvious.
And that complex is unstable and even has the possibility of failure.
In a complexometric titration, hydrogen ions are generally released. Therefore, as
the reaction proceeds, the pH of the solution changes continuously.This seriously
affects the color development of the indicator. Therefore,
a certain amount of buffer solution should be added during titration to control the pH
value of the solution in a certain range. Only in this way can the experimental
phenomena be obvious and the results be accurate.
-
EDTA Series
- EDTA Acid
- EDTA-Na2
- EDTA-Na4
- EDTA-CaNa2
- READ MORE...
-
Organophosphoric
- ATMP
- BHMTPMPA
- DTPMPA
- EDTMPA
- HEDP
- READ MORE...
-
Other Chelating Agent
- DTPA
- NTA.Na3
- DTPA.Na5
- READ MORE...


